Time for a new gold standard in HIV viral load monitoring
 November 29, 2012
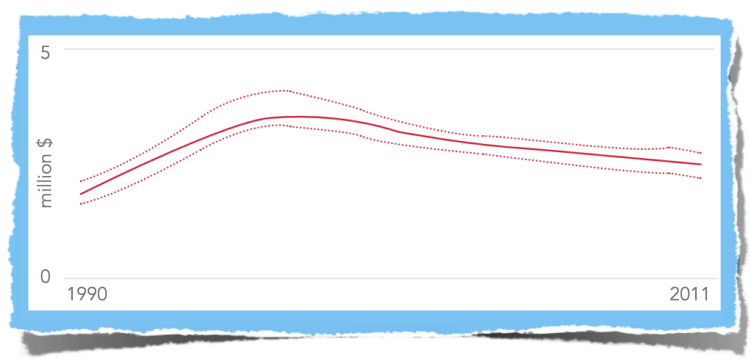
November 29, 2012 This past year has brought more good news in the battle against HIV/AIDS with UNAIDS stating, “On the cusp of the fourth decade of the AIDS epidemic, the world has turned the corner—it has halted and begun to reverse the spread of HIV.” UNAID’s 2012 report cited 700,000 fewer new HIV infections in 2011 than in 2001. AIDS-related deaths have been reduced by one-third in the past six years. And access to antiretroviral therapy (ART) continues to grow at unprecedented rates. But as the battle against HIV enters a new phase, it introduces new challenges to the healthcare community, particularly with regard to diagnostics. In response, the World Health Organization and UNITAID have dubbed the next ten years, “the decade of diagnostics.” In their session at AIDS 2012 in Washington D.C. they emphasized the important role that cheaper, simplified diagnostics must play in the next phase of the campaign to stem the HIV pandemic. This emphasis is redefining the role of HIV viral load testing in treatment and is placing new demands on how these tests are conducted.
Number of people newly infected with HIV, Global, 1990-2011
For decades the gold standard for HIV viral load diagnostics have been RNA-based tests. But in this new diagnostic landscape I see centralized RNA-based testing rapidly losing relevance to tools that are better suited to meet the diagnostics challenges that we see today in both the developed and developing world. Most notable among these are: a) the need to scale HIV viral load monitoring in step with the burgeoning number of men, women and children entering treatment, b) managing the rise in drug-resistant HIV strains that accompany greater access to ARV treatment and c) address the diagnostic needs of infants born to HIV-positive mothers.
In low-to-middle income countries, access to HIV viral load testing has become a more critical issue given the recent increase in access to ART. According to the World Health Organization (WHO), there was a 20-fold increase in the number of people receiving ART in developing countries between 2003 and 2011, and a 20% increase in just one year (from 6.6 million in 2010 to more than 8 million in 2011). The rapid increase in access to Antiretroviral drugs (ARV) has triggered a corresponding increase in the need to monitor those receiving treatment. This helps to ensure the virus is being suppressed and helps the doctor know when the patient needs to be switched to a new treatment regimen.
Originally developed for use in North America and Europe, RNA-based tests are proving impractical for decentralized use in low-to-middle income countries. Around 70% of the world’s HIV population live in sub-Saharan Africa. As a result, district hospitals and clinics outside the capital have to either send blood samples away to a central reference hospital or, more likely, forgo HIV viral load monitoring altogether. In light of this, it seems the gold standard is shifting in favor of a HIV viral load monitoring solution that can deliver the same reliability in a decentralized model with testing conducted near-patient.
This has created a flurry of innovation in the HIV viral load POC testing arena. Maurine Murtagh has identified 13 different entrants in this area in the 2nd edition of UNITAID Diagnostic Technology Landscape Report. Of the options available today, Reverse Transcriptase (RT)-based testing seems to offer the most plausible solution on several fronts. First, RT is a very stable marker since it is not affected by mutation and is always present when the HIV virus is replicating. Since RT-based tests do not target a specific nucleic acid sequence, they are able to quantify all types and subtypes of HIV, including new strains, without any modification to the test. RT-based tests have historically been significantly less expensive than RNA tests both in terms of start-up and running costs. Further, the RT platform has an unmatched track record among this next generation of HIV viral load tests. It has been in the field for over a decade with more than 40 peer-reviewed journal articles and over 350,000 tests run. Several studies over the past decade have compared ExaVir™ Load to the gold standard PCR tests and all have found excellent correlation with RNA-based tests.
The benefits of RT-based HIV viral load testing go beyond resource-limited settings. In the developed world, HIV viral load monitoring is a main line of defense against the rise in drug resistant strains of HIV. Eric Rubin, professor of immunology and infectious diseases at HSPH put it eloquently, "Drug resistance is the product of success: With treatment, we have drug resistance." Since ARV treatment has been more prevalent in developed countries, resistance has mainly been a problem for these nations. For instance, a recent study in San Francisco revealed that 60 percent of new HIV infections are drug resistant. One of the key factors in stemming this tide is early detection of treatment failure through HIV viral load monitoring of all HIV positive patients. Since healthcare systems the world over are straining to manage budgets, a more cost-effective decentralized HIV viral load monitoring solution may benefit developed nations as much as it does low-to-middle income countries.
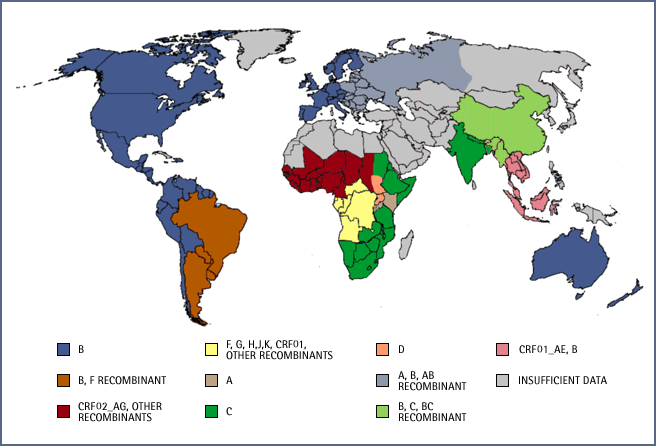
In areas where the subtype of the individual may be unknown RT-based testing provides additional advantages. This has not been much of a concern in the US where the vast majority of HIV-1 infections are subtype B—98 percent according to some surveys. But an article from CAP Foundation asserts that it may be time for the US to “catch up to what’s happening in Tanzania and elsewhere in Africa. Specifically, HIV-1 subtypes common in Africa may be making inroads in the United States, as they have in Europe.” Of the testing options available, only RT-based testing is able to detect any HIV activity without modifying the test — including new HIV strains.
World map of Global distribution of HIV-1 strains
Lastly, with half of the world’s HIV population being women and many of them of child-bearing age, there has been increased focus in recent years on mother-to-child transmission. Here too we see great strides have been made with 57% of HIV positive pregnant woman living in low-and middle-income countries receiving treatment in 2011. One persistent problem has been the early infant diagnosis (EID) since standard rapid tests won’t work on newborns. This is another area where RT-based testing has been found to convey an advantage. Over the past year more studies have confirmed that in addition to RT-based EID solutions being significantly less expensive than RNA-based tests, they are also able to detect and quantify HIV infection in infants more reliably and at a much younger age.
This World AIDS Day, as Cavidi celebrates the 25th Anniversary of our RT-technology, I’m pleased to report that we are making steady progress on three fronts to address the challenges above. First, we continue to support the increasing uptake of our manual ExaVir Load HIV viral load monitoring test which is increasing access to affordable HIV viral load testing around the world. Second, we have made excellent progress developing a new automated platform for near patient HIV viral load monitoring. The platform design is now entering final stages of prototype development and testing. And third, over this past year we have initiated further studies into the development of an RT-based EID test. I look forward to sharing more details on these exciting developments over the next year.
New challenges require new solutions. As we enter the decade of diagnostics I hope to see a new gold standard emerge that will make HIV viral load testing more accessible and reliable. My team will do their part as they continue to bring innovative RT-based diagnostics to the world in 2013 and beyond.

John Reisky de Dubnic
CEO
Cavidi